
| Copyright © Queen's Printer, Victoria, British Columbia, Canada |
Licence
Disclaimer |
||
|
|||
B.C. Reg. 278/2018, deposited December 14, 2018, under the PILL PRESS AND RELATED EQUIPMENT CONTROL ACT [sections 27 (2) and 30] and the OFFENCE ACT [section 132 (2)]. Order in Council 793/2018, approved and ordered December 14, 2018.
On the recommendation of the undersigned, the Lieutenant Governor, by and with the advice and consent of the Executive Council, orders that, effective January 15, 2019,
(a) the Pill Press and Related Equipment Control Act, S.B.C. 2018, c. 24, except the following sections, is brought into force:
(i) section 1 as it enacts the definition of “authorized health professional”;
(ii) sections 2 (1) (a) and (b) and (2), 5 (2) (d) and (e), 17 (1) (a) and (b), 20 (2) (c) (ii) and (3) (b) and (c) and 27 (2) (a) and (c),
(b) the Pill Press and Related Equipment Control Regulation as set out in Appendix 1 is made, and
(c) the Violation Ticket Administration and Fines Regulation, B.C. Reg. 89/97, is amended as set out in the attached Appendix 2.
— D. EBY, Attorney General; M. FARNWORTH, Minister of Public Safety and Solicitor General; J. SIMS, Presiding Member of the Executive Council.
Appendix 1
PILL PRESS AND RELATED EQUIPMENT CONTROL REGULATION
1 In this regulation:
“Act” means the Pill Press and Related Equipment Control Act;
“drug establishment licence” means an establishment licence issued under the Food and Drug Regulations (Canada);
“drug establishment licence number” means the unique alphanumeric identifier assigned to a drug establishment licence;
“equipment registry number” means the unique alphanumeric identifier assigned by the registrar to a piece of controlled equipment for the purpose of a registry established under section 14 (1) (a) of the Act;
“fabricator” means a person who holds a drug establishment licence or a site licence;
“registration number” means the unique alphanumeric identifier assigned to a registration;
“site licence” means a site licence issued under the Natural Health Products Regulations (Canada);
“site licence number” means the unique alphanumeric identifier assigned to a site licence;
“waiver number” means the unique alphanumeric identifier assigned to a waiver.
2 A pharmaceutical mixer or blender is not controlled equipment if the pharmaceutical mixer or blender is used to make tablets or capsules
(a) in the course of compounding a drug by a person authorized to do so under the Health Professions Act or the Pharmacy Operations and Drug Scheduling Act, or
(b) by an individual for personal use.
Part 2 – Dealing with Controlled Equipment
Ownership and possession if licence suspended or cancelled
3 (1) If the drug establishment licence or site licence of a fabricator is suspended, the fabricator is authorized, during the period of the suspension, to continue to own and possess all controlled equipment that was owned or possessed by the fabricator on the suspension date.
(2) If a drug establishment licence or site licence is cancelled, the former fabricator is authorized to continue to own and possess all controlled equipment that was owned or possessed by the former fabricator on the cancellation date as follows:
(a) if the former fabricator applies for a waiver or registration within 30 days after cancellation, the former fabricator may own and possess the controlled equipment until
(i) the waiver or registration is granted, and afterwards in accordance with the limits and conditions of the waiver or registration, or
(ii) the waiver or registration is refused, and afterwards until the date set by the registrar as the date by which the controlled equipment must be destroyed, as required by the registrar;
(b) if the former fabricator does not apply for a waiver or registration within 30 days after cancellation, on the expiry of that 30-day period.
Information to be collected on sale
4 (1) An authorized seller must collect from an intended purchaser who is not an individual the following information respecting the purchaser’s identity:
(a) the name under which the purchaser is registered as a corporation or other body under an enactment of any jurisdiction, and the registration number;
(b) the name under which and the civic address at which the purchaser ordinarily carries on business;
(c) the legal name, business telephone number and email address of the person responsible for making the purchase on behalf of the purchaser.
(2) An authorized seller must collect from an intended purchaser who is an individual the following information respecting the purchaser’s identity:
(a) the purchaser’s legal name, civic address, telephone number and email address;
(b) the unique alphanumeric identifier assigned to a record of identity that
(i) is issued to the purchaser by a government,
(ii) shows the purchaser’s photograph and legal name, and
(iii) is not expired;
(c) a description of the type of record referred to in paragraph (b).
(3) An authorized seller must collect from an intended purchaser who is an authorized owner the information set out in one of the following paragraphs, as applicable, respecting the purchaser’s authority to own controlled equipment:
(a) if the purchaser is a fabricator,
(i) the name shown on the fabricator’s drug establishment licence or site licence, and
(ii) the fabricator’s drug establishment licence number or site licence number and the licence expiry date;
(b) if the purchaser holds a waiver, the purchaser’s legal name and waiver number;
(c) if the purchaser holds a registration, the purchaser’s legal name and registration number.
(4) For the purposes of subsection (3) (a), if a purchaser holds both a drug establishment licence and a site licence, the authorized seller must collect the information referred to in that paragraph in respect of both licences.
5 (1) In this section:
“cash card” means a card or other device that
(a) can be used to obtain cash or acquire goods or services, and
(b) is issued by a payday lender within the meaning of the Business Practices and Consumer Protection Act to the borrower of a payday loan within the meaning of that Act instead of advancing cash or transferring money to the borrower or to the order of the borrower;
“prepaid purchase card” means
(a) a card, written certificate or other voucher or device with a monetary value that is issued or sold to a person in exchange for the future supply of goods or services to a consumer, or
(b) a gift card and gift certificate;
“virtual currency” means a digital representation of value that is used as a medium of exchange, unit of account or store of value, but is not legal tender and is not regulated.
(2) An authorized seller must not receive, as any part of the consideration for the sale of controlled equipment, a cash card, prepaid purchase card or virtual currency.
6 An authorized seller must include in a record of sale made for the purposes of section 4 (5) of the Act all of the following:
(a) the date on which the sale was made;
(b) a description of the controlled equipment that was sold, including the type of equipment and its make, model and serial number;
(c) the authorized seller’s registration number;
(d) the method of payment used to purchase the controlled equipment;
(e) if known,
(i) the civic address at which the purchaser will ordinarily store the controlled equipment, and
(ii) whether storage will be in a dwelling house.
Information to be given on acquisition
7 An authorized owner must include all of the following in a notice given to the registrar under section 5 (2) (a) and (4) (c) of the Act:
(a) the information described in section 4 of this regulation that applies to the authorized owner, as if the authorized owner was a purchaser under that section;
(b) a description of the controlled equipment, including the type of equipment and its make, model and serial number;
(c) if the controlled equipment was acquired from an authorized seller, the authorized seller’s legal name and registration number;
(d) if the controlled equipment was not acquired from an authorized seller, the seller’s legal name and civic address;
(e) the date on which the authorized owner acquired the controlled equipment, if that date is on or after January 15, 2019;
(f) the civic address at which the controlled equipment will ordinarily be stored.
Information to be given on change of storage location
8 An authorized owner must include all of the following in a notice given to the registrar under section 5 (2) (b) and (4) (c) of the Act:
(a) the equipment registry number that applies to the controlled equipment;
(b) the civic addresses at which the controlled equipment was formerly ordinarily stored and is to be ordinarily stored;
(c) the date on which the controlled equipment was moved between the addresses referred to in paragraph (b).
Information to be given on loss, theft or destruction
9 An authorized owner must include all of the following in a notice given to the registrar under section 5 (2) (c) and (4) (c) of the Act:
(a) the equipment registry number that applies to the controlled equipment;
(b) a description of the controlled equipment, including the type of equipment and its make, model and serial number;
(c) the date on which, and the circumstances under which, the controlled equipment was lost, stolen or destroyed;
(d) the police file number, if any.
Information to be given on licence suspension or cancellation
10 An authorized owner must include all of the following in a notice given to the registrar under section 5 (2) (f) and (4) (c) of the Act:
(a) the name shown on the fabricator’s, or former fabricator’s, drug establishment licence or site licence, as applicable;
(b) the drug establishment licence number or site licence number, as applicable, of the suspended or cancelled drug establishment licence or site licence;
(c) if the drug establishment licence or site licence was cancelled and replaced for administrative reasons only, the drug establishment licence number or site licence number, and the licence expiry date, of the replaced drug establishment licence or site licence.
Information to be given on charge or conviction
11 An authorized seller must include all of the following in a notice given to the registrar under section 5 (2) (g) and (4) (c) of the Act:
(a) the authorized seller’s legal name and registration number;
(b) the name and provision of the enactment under which the authorized seller was charged or convicted;
(c) the date on which the charge was laid or the conviction entered, as applicable.
Exemption for moulds and punches
12 An authorized owner of a mould or punch described in paragraph (b) of the definition of “controlled equipment” is exempt from the notification requirement imposed under section 5 (2) (a), (b) and (c) of the Act.
Period for which records must be kept
13 (1) An authorized owner must keep a record referred to in section 6 (1) (c) of the Act
(a) for the period during which the authorized owner owns or possesses the controlled equipment that is the subject of the record, and
(b) for at least 2 years after the date on which the authorized owner no longer owns or possesses the controlled equipment.
(2) Without limiting subsection (1), an authorized seller must keep a record made under section 4 (5) (a) of the Act for at least 2 years after the date on which the authorized seller receives confirmation from the registrar that the registrar has received all records and information required under section 4 (5) (c) of the Act.
Checks of registration applicants
14 The following are prescribed for the purposes of section 7 (4) (a) of the Act:
(a) a criminal record check;
(b) a police information check;
(c) a correctional service information check.
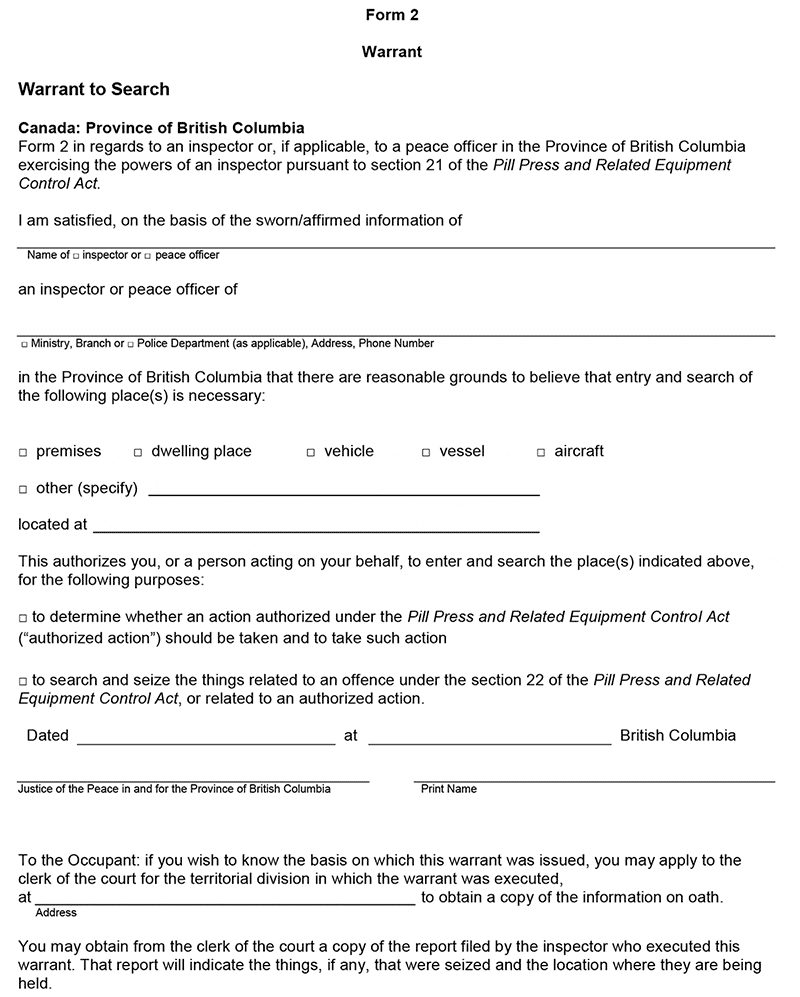
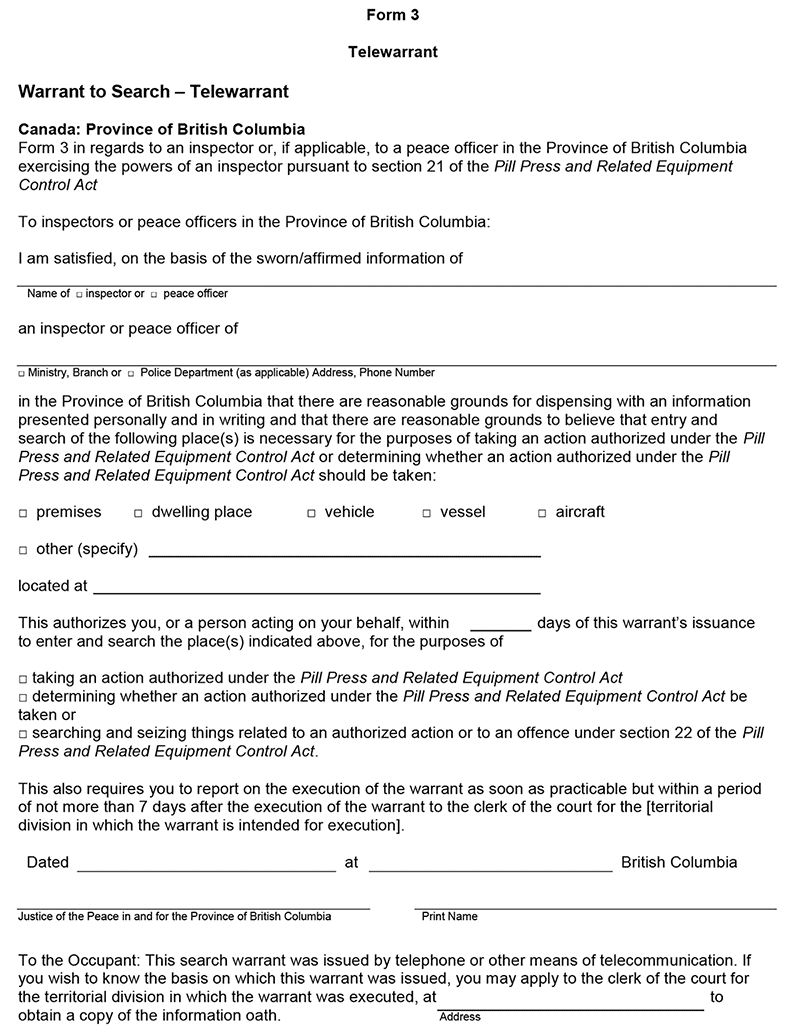
15 (1) An application for a warrant under section 21 of the Act may be made by submitting information on oath in the form set out as Form 1 of the Schedule.
(2) A warrant may be issued in the form set out as
(a) Form 2 of the Schedule, if the application was made in person, or
(b) Form 3 of the Schedule, if the application was made by telephone or other means of telecommunication.
Transition of controlled activities
16 (1) For the purpose of section 28 (2) of the Act, a person who owned controlled equipment before January 15, 2019 and is authorized to continue to own the controlled equipment on that date must give to the registrar, before July 1, 2019, the records described in section 7 (a), (b) and (f) of this regulation.
(2) For the purpose of section 28 (3) of the Act, a person who is in the business of selling controlled equipment before January 15, 2019 must apply for a registration before April 1, 2019 for that provision to apply.
(3) For the purpose of section 28 (5) of the Act, a person who owned controlled equipment before January 15, 2019 but is not authorized to continue to own the controlled equipment on that date must apply for a waiver before April 1, 2019 for that provision to apply.
(section 15)



Appendix 2
1 Schedule 1 of the Violation Ticket Administration and Fines Regulation, B.C. Reg. 89/97, is amended
(a) in column 2 of Item 1B by adding the following paragraph:
(m.01) Pill Press and Related Equipment Control Act , and
(b) by adding the following items as indicated:
| Item | COLUMN 1 Enforcement Officer |
COLUMN 2 Enactments for Which a Violation Ticket, Other Than an eTicket, or an Appearance Notice May Be Issued |
COLUMN 3 Enactments for Which an Appearance Notice May Be Issued but a Violation Ticket May Not Be Issued |
|---|---|---|---|
| 33 | A person appointed under section 16 of the Pill Press and Related Equipment Control Act as an inspector | Pill Press and Related Equipment Control Act | |
| 34 | A person authorized under section 17 of the Pill Press and Related Equipment Control Act to exercise powers or perform duties as if the person were an inspector | Pill Press and Related Equipment Control Act | |
2 Schedule 2 is amended by adding the following items as indicated:
| 1 Provision |
2 Contravention |
3 Fine |
4 Victim Surcharge Levy |
5 Ticketed Amount |
|
|---|---|---|---|---|---|
| Pill Press and Related Equipment Control Act | |||||
| section 2 (1) | Own, possess or use controlled equipment without authorization | $500 | $75 | $575 | |
| section 2 (1) (c) | Own, possess or use controlled equipment contrary to the limits and conditions of an authorization | $500 | $75 | $575 | |
| section 2 (1) (d) | Own, possess or use controlled equipment contrary to the limits and conditions of a waiver or registration | $500 | $75 | $575 | |
| section 2 (1) (e) | Own, possess or use controlled equipment contrary to prescribed limits and conditions | $500 | $75 | $575 | |
| section 4 (1) | Sell controlled equipment in contravention of a registration, enactment or court order | $500 | $75 | $575 | |
| section 4 (2) | Sell controlled equipment to a person in British Columbia who is not an authorized owner | $500 | $75 | $575 | |
| section 4 (3) | Fail to collect prescribed information from intended purchaser | $500 | $75 | $575 | |
| section 4 (4) | Receive cash or a type of prescribed consideration as consideration for sale of controlled equipment | $250 | $38 | $288 | |
| section 4 (5) (a) (i) | Fail to make a record of sale in the required form | $250 | $38 | $288 | |
| section 4 (5) (a) (ii) | Fail to include required information in a record of sale | $250 | $38 | $288 | |
| section 4 (5) (b) | Fail to give purchaser a record of sale | $250 | $38 | $288 | |
| section 4 (5) (c) | Fail to give registrar required records and information within the required time | $250 | $38 | $288 | |
| section 5 (1) | Fail to ensure that controlled equipment is stored securely | $250 | $38 | $288 | |
| section 5 (2) (a) | Fail to notify registrar of import, construction, manufacture, assembly or purchase of controlled equipment | $500 | $75 | $575 | |
| section 5 (2) (b) | Fail to notify registrar of a change in location where controlled equipment is ordinarily stored | $500 | $75 | $575 | |
| section 5 (2) (c) | Fail to notify registrar if controlled equipment is lost, stolen or destroyed | $500 | $75 | $575 | |
| section 5 (2) (f) | Fail to notify registrar if authority to manufacture a drug or natural health product is suspended or cancelled | $500 | $75 | $575 | |
| section 5 (2) (g) | Fail to notify registrar if charged with or convicted of a relevant offence | $500 | $75 | $575 | |
| section 6 (2) (a) | Fail to keep records in accessible location | $500 | $75 | $575 | |
| section 6 (2) (b) | Fail to produce records as required | $500 | $75 | $575 | |
| section 22 (3) (a) | Fail to comply with an order of an inspector | $500 | $75 | $575 | |
| section 22 (3) (c) | Knowingly provide false or misleading information | $500 | $75 | $575 | |
Copyright © 2019: Queen's Printer, Victoria, British Columbia, Canada